
1
Step 1
Quick pre-screening call with our patient engagement team to check which studies are suitable.

Participate in groundbreaking research and access new treatments. Your involvement supports medical advancements while you benefit from expert care.
Register today for more information.
Clinical trials help researchers test new treatments and improve healthcare for people with dementia and cognitive impairment. These studies are essential for finding better ways to prevent, diagnose, and treat medical conditions.
In 2023/24, over a million people in the UK took part in health and social care research, according to the National Institute for Health and Care Research (NIHR). Every study brings us closer to new treatments – and possibly even a cure.
By joining a clinical trial, you can play a vital role in shaping the future of medicine.
Our clinics located throughout the UK are set up for your comfort with experts and consultants to walk you through the clinical trial process.
We have clinics in London, Guildford, Plymouth, Birmingham, Winchester and Bristol.
We’re thrilled to be a preferred site within the PPD Select Partnership Programme, a key initiative of Thermo Fisher Scientific’s PPD clinical research business, a global leader in clinical research.
This partnership opens up exciting opportunities for our patients and participants. By working with PPD’s Strategic Site Collaborations team, we’re able to offer access to cutting-edge clinical trials and provide the highest quality of care across our ten locations in the UK and USA.
We’re committed to continuously enhancing our services and providing you with the best research opportunities available.
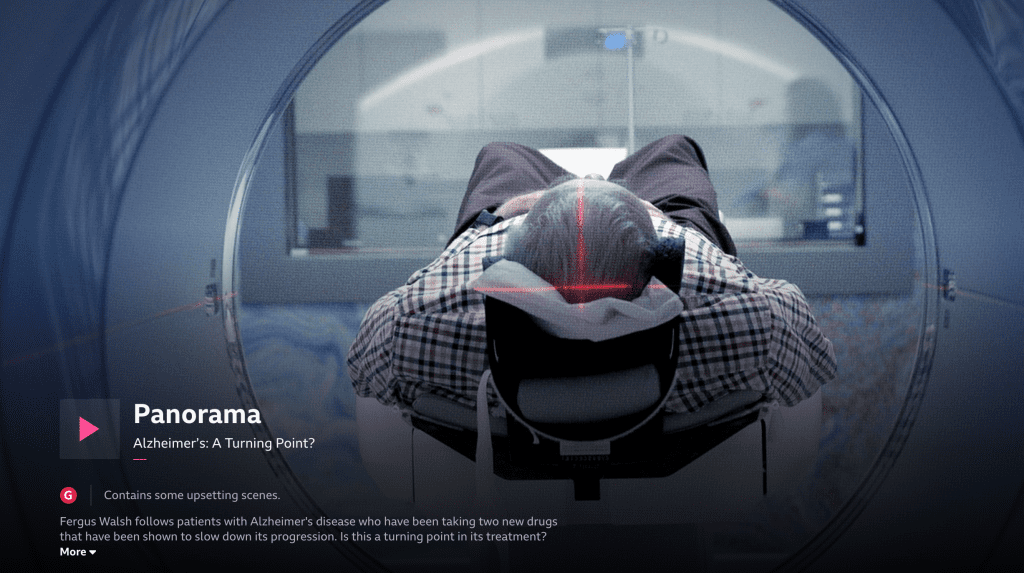
Re:Cognition Health has recently been featured on BBC One’s Panorama, “Alzheimer’s: A Turning Point?”. This follows the journey of volunteers participating in clinical trials, some from Re:Cognition Health’s UK centres, testing new-generation medications designed to slow down the progression of the disease. For those in the UK, you can still watch this on BBC iPlayer.
Dr Emer MacSweeney, CEO and Founder of Re:Cognition Health, discusses the latest medications available for Alzheimer’s disease. In this video, we hear from clinical trial participants Mike and David, as well as trial partner Cheryl, who share how participating in clinical trials for these new treatments has positively impacted their lives.
Explore our blogs, news, and resources as you prepare for the trials.
Sign Up for Our Newsletter
Get our latest news, podcasts, insights, and expert knowledge
"*" indicates required fields