Re:Cognition Health proud to be part of ground-breaking clinical trial
Dr Emer MacSweeney, CEO at Re:Cognition Health shares her excitement about the potential success of Aducanumab, which has been in the news this week as the first drug to slow down Alzheimer’s disease progression.
Re:Cognition Health so proud to have been part of the recent international clinical trial providing the opportunity for individuals in the early stages of Alzheimer’s, to enrol on Biogen’s Aducanumab study and thereby gain access to this new medication, which could be life-changing for so many people today and in the future.
Re:Cognition’s team of brain and mind experts are delighted to learn that Biogen are seeking regulatory approval in the USA for Aducanumab. This new medication targets and the harmful amyloid protein which builds in the brain, eventually destroying brain cells and causing Alzheimer’s disease. Although this is not a cure for Alzheimer’s, this is a hugely encouraging and successful result for clinical trials; proving that research is imperative and never in vain.
Why is this trial so exciting?
Today, there are no treatments available on the market to slow the relentless progression of Alzheimer’s. Aducanumab, if made available on the market, will be the first; whilst cautious optimism is appropriate, news this week about Aducanumab is the most exciting news ever about a new type of effective treatment for Alzheimer’s. Biogen, the pharmaceutical company that conducted this trial, will be seeking regulatory approval in the US, with hopes to extend to Europe.
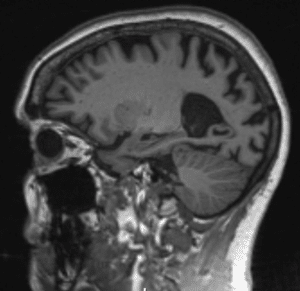
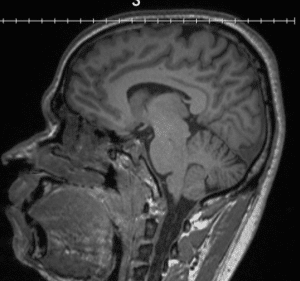
Brain scan of someone with Alzheimer’s Disease (left) note shrunken appearance compared to normal brain (right)
Clinical trials push the limits of our understanding
Re:Cognition Health, also undertakes many more, similar, international clinical trials at 4 of its Centres in the UK and at its first Center in the USA.
Every year more and more people learn about the opportunity provided to them, now, through enrolling on clinical trials and being the first to gain access to the many new medications, like Aducanumab, which are being designed specifically to slow or ideally halt progression of memory loss and other cognitive symptoms, due to Alzheimer’s Disease. Without these new generation treatments, symptoms progress and the earlier people can get access to these new medications, to slow further progression of memory loss, the more chance they have of beating this devastating condition.
According to the National Institute for Clinical Research, over 870,000 people participated in clinical trials in the UK in 2018 for treatments in the health and social care sector. With every clinical study conducted we become closer to understanding each given condition and to finding new treatments and cures for current and future generations. Unfortunately, only a very small percentage of those with mild memory problems are even diagnosed, let alone get early access to these new treatments.
This is particularly alarming as Dementia is the only cause of death still on the rise and Alzheimer’s disease is the most common cause. Every 3 seconds someone in the world develops dementia and without effective treatments, 1 in 3 people born today will die with it, making dementia today’s biggest global socio-economic and health care crisis.
The potential regulation of Aducanumab signifies the beginning of a big change for the future. We hope it will give confidence to millions worried about their memory, the courage to seek an early accurate diagnosis and the opportunity to enrol on clinical trials; so they too can gain access to new generation medications, designed to change, today, their own future and tomorrow the future of the next of their family.
Get involved in a clinical trial
For further information on clinical trials and what is involved, please visit https://recognitionhealth.com/clinical-trials-at-recognition-health/ or call our team of experts on 02033553536.
 Visit our USA website
Visit our USA website